Astro 103 - Lecture 4

 Lectures Lectures  |
Lecture page |
|

 Lectures Lectures  |
Lecture page |
|
 1/
1/
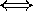 shorter wavelengths
shorter wavelengths
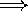 higher pitch
higher pitch
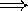 bluer color
bluer color
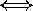 longer wavelengths
longer wavelengths
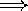 lower pitch
lower pitch
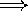 redder color
redder color
 ) or wavelength (
) or wavelength (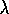 )
)
When does it happen?
and ...
there is some component of radial motion
between the source and observer.
(some portion of the motion is straight
towards you or away from you)
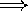 the frequency goes down
the frequency goes down
 the wavelength appears to get longer
the wavelength appears to get longer
 the frequency goes up
the frequency goes up
 the wavelength appears to get shorter
the wavelength appears to get shorter
Kirchhoff's laws
Analogy with gravity and orbits: ![]()
electrons as the planets
the energy levels of atoms and molecules are discrete
(electrons can't be in just any 'orbit' around the nucleus)
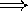 Whenever there is an energy
transition, light (a photon) is either given off (emitted), or
absorbed by the electron.
Whenever there is an energy
transition, light (a photon) is either given off (emitted), or
absorbed by the electron.
Emission: electron emits photon, and moves to a lower (less
energetic) orbit
![]()
Absorption: electron absorbs a photon, and moves to a higher (more energetic) orbit.
temperature and density - relative strengths of various emission or absorption lines
distance -
 Lectures Lectures  |
Lecture page |
|