Astro 103 - Lecture 3

 Lectures Lectures  |
Lecture page |
|

 Lectures Lectures  |
Lecture page |
|
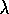 ) or frequency (
) or frequency ( )
)
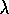 = c /
= c / 
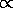

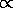 1/
1/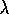
What do we see on Earth from the sun?
![]()
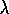 peak =
2900 / T (peak wavelength)
peak =
2900 / T (peak wavelength)
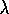 in
units of microns
in
units of microns
 T4
T4
What you measure depends on how you measure it!
The same idea is true for all fundamental ``particles'' such as electrons, protons, neutrons, etc. Sometimes they behave like waves; other times they behave like particles.
 Lectures Lectures  |
Lecture page |
|