- Emission and Absorption
- Analogy with gravity and orbits:
- atomic nucleus as the sun
- electrons as the planets
- BUT! quantum mechanics:
- energy comes in discrete packets or quanta
- the energy levels of atoms and molecules are discrete
(electrons can't be in just any 'orbit' around the nucleus)
 Whenever there is an energy
transition, light (a photon) is either given off (emitted), or
absorbed by the electron.
Whenever there is an energy
transition, light (a photon) is either given off (emitted), or
absorbed by the electron.
Emission: electron emits photon, and moves to a lower (less
energetic) orbit
Absorption: electron absorbs a photon, and moves to a higher (more
energetic) orbit.
If the photon has enough energy, it may even 'ionize' the atom, i.e.
give the electron enough energy to 'escape' from the nucleus.
 Lectures
Lectures 
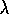
 1/
1/
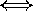 shorter wavelengths
shorter wavelengths  higher pitch
higher pitch  bluer color
bluer color  longer wavelengths
longer wavelengths  lower pitch
lower pitch 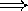 redder color
redder color  ) or wavelength (
) or wavelength (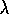 )
) 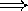 the frequency goes down
the frequency goes down
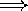 the wavelength appears to get longer
the wavelength appears to get longer
 the frequency goes up
the frequency goes up
 the wavelength appears to get shorter
the wavelength appears to get shorter
 Whenever there is an energy
transition, light (a photon) is either given off (emitted), or
absorbed by the electron.
Whenever there is an energy
transition, light (a photon) is either given off (emitted), or
absorbed by the electron.